Understanding Electrolyte Product Comparisons
Understanding Electrolyte Product
Comparisons
Summary:
Electrolyte product labels inconsistent.
Most common unit of measurement is mmol which is a measurement of the actual molecules of the different ingredients.
Ingredients listed on a label often do not indicate the actual concentration of ingredients in the electrolyte solution.
Ingredients dissociate into base components when added to water which has a profound affect on the acid-base balance of the solution.
Electrolyte solutions have ‘must have’ components to be effective in restoring a calf’s electrolyte balance.
There are tremendous variations in the formulations of oral electrolyte solutions currently available in the NZ.
Electrolyte product comparisons show up in articles and publications from time to time and are typically based on information found on the label of each product. Although probably unintentional, these comparisons usually contain a fair amount of incorrect or misleading information. These profiles may be convenient and are intended to be informative, but caution needs to be exercised when using these comparisons.
To begin with, the product profiles in these comparisons use values that have been calculated from the actual numbers of molecules of the different ingredients that make up each electrolyte product. What?! If you’ve never studied or forgotten school chemistry, don’t panic, you are in good company.
It’s actually the use of these principles that leads to problems with electrolyte evaluations and comparisons – not just in published comparisons, but across the board. The intent of this article is to shed some light on how these problems are generated, and how to avoid the pitfalls they create.
Millimole:
The term or unit of measure most commonly used in electrolyte evaluations is millimole, abbreviated to mmol. Simply put, this is a measure of the concentration of a substance, such as sodium, chloride, or glucose that is dissolved in a solution. The term osmolarity is often used when talking about mmol concentrations.
To evaluate an electrolyte product, we need to work with the solution the calf actually consumes, not just what’s in the package on the shelf. We start with the dry product, add water according to the label instructions and then evaluate. This is all figuratively speaking, of course — we do this on paper, not in an actual bucket. The label provides a list of ingredients and a guaranteed analysis.
The Ingredient List is an accounting of the ingredients used to make the product, whereas the Guaranteed Analysis states the concentrations of various ingredients or specific nutrients provided in the dry product. Sometimes the details in the Guaranteed Analysis can be a bit skimpy, especially if a manufacturer is protecting a proprietary formula. That’s understandable since they may have a significant investment in the product and don’t want to give the formula away. In such cases, a few phone calls or emails to manufacturers may be necessary to provide sufficient detail for each product.
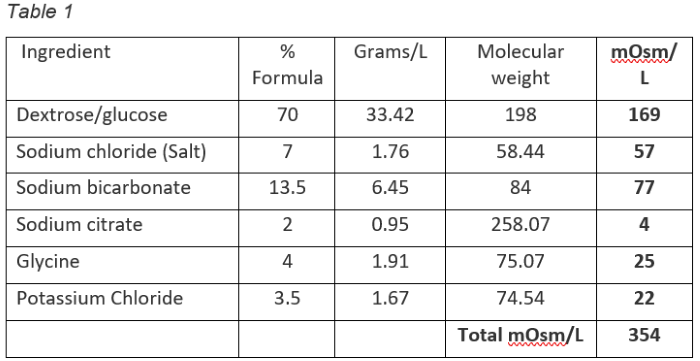
The example electrolyte product shown in Table 1 below contains six ingredients which are listed in the left-hand column. The right-hand column shows the mOsm/litre concentration of each ingredient in the final solution. (Sometimes shown as mmol/L or mml/L) The other columns are mathematical steps along the path to our mmol objectives.
Electrolyte example
This is the standard approach to evaluating electrolytes. There is, however, one slight problem. Several of the ingredients (sodium chloride, sodium citrate, sodium bicarbonate and potassium chloride) don’t exist in the electrolyte solution that the calf drinks. Once these substances come into contact with water, they dissociate into their base components. In this case, we’re left with sodium, chloride and potassium (which are called strong ions) as well as citrate and bicarbonate.
A good indicator of whether or not an electrolyte ingredient will dissociate, is the presence of one or more strong ions. Sodium, chloride, potassium, calcium and magnesium are all strong ions. It’s pretty easy to identify which ingredients contain strong ions since their presence is indicated in the ingredient name.
This dissociation has profound effects on the acid-base balance in the body as well as having profound effects on the osmolarity of the solution. For example, the actual impact of the sodium chloride in this formula on osmolarity is 114 mOsml/L, not 57 mOsm/L as shown in Table 1. Sodium citrate, sodium bicarbonate and potassium chloride actually contribute 16, 154 and 44 mOsm/L, respectively. Dissociation of sodium citrate yields four molecules (three sodium and one citrate), whereas sodium chloride, sodium bicarbonate and potassium chloride dissociate into two molecules each. Glucose and glycine stay the same since they don’t dissociate.
Some electrolyte product comparisons go further and provide a total mOsm/L value for the different electrolyte products being compared. These values can be quite inaccurate and misleading. Our example electrolyte in Table 1 shows a total mOsm/L value of 354. The actual osmolarity of the product is 522 mOsm/L as shown in Table 3. So be cautious about using these numbers.
Although Table 1 and 2 seems logical from the standpoint that it provides a ledger for the ingredients specified in the formula, Table 3 accounts for ingredient dissociation and provides a more accurate description of the electrolyte solution consumed by the calf. It allows for correct assessments of osmolarity, and results in a higher degree of accuracy when evaluating electrolytes.
Osmolarity:
“Osmolarity” expressed as mOsml/L is simply a term used to measure the concentration of particles in a solution. Thus, the higher the concentration of a solution, the higher the osmolarity will be. Commercially available oral electrolyte solutions for use in calves range from 300 mOsm/L (called “isotonic” because this is equal to the osmolarity of blood) to 750 mOsm/L, (which would be considered very hypotonic or concentrated). Simply stated, the higher the osmolarity of a product the more concentrated it is or generally the more electrolytes and energy (glucose) the product contains. However too high an osmolarity can cause problems.
The maximum osmolarity normally found in the intestine tract is about 600 mOsm/L and any electrolyte solutions with above this should be avoided as they could worsen the damage that has been done to the digestive system and cause abomasa bloat.
When a calf is dehydrated and suffering from scours, the digestive system becomes compromised from decreased absorption. Sodium, chloride, and potassium are all lost in the faeces of calves with diarrhoea. Sodium is the most important of these and most research suggests a level of 90-145 mmol/L is necessary to correct dehydration. However, sodium and accompanying water absorption from the small intestine will only occur if there is glucose or an amino acid such as glycine, alanine, or glutamine that the sodium can join with and cross into the cells in the gut.
The ratio of glucose to sodium present in an oral electrolyte solution should fall somewhere between 1:1 and 3:1. The process of absorbing sodium helps rehydrate the calf by replenishing total body fluids. Volatile fatty acids such as acetate and propionate are also known to increase intestinal absorption of sodium.
With dehydration, potassium is lost in the faeces and urine so calves may experience a profound loss of body potassium stores. A common clinical sign in calves with chronic diarrhoea is extreme muscle weakness due in large part to this loss of potassium. Oral electrolyte products should contain between 10-30mmol/L of potassium.
Strong Ion (SID):
Strong ion (SID) A relatively new theory called the “strong ion theory” encourages the use of products that deliver an excess of strong cations (sodium and potassium) relative to the concentration of strong anions (chloride) in order to help correct a portion of the acid-base balance in the blood. This “strong ion difference” or “SID” is calculated as follows: [Na+] + [K+] –[Cl-]= SID and should fall in the range of 60-80 in an oral electrolyte product. Chloride should be present in the range of 40-80 mmol/L. Concentrations at the lower end of the suggested range will beneficially increase the SID.
Alkalinizing agents:
Virtually all calves with diarrhoea have a decrease in their blood pH as compared to normal. This acidosis is largely responsible for the abnormal clinical signs seen in these animals including loss of suckle reflex, depression, inability to stand, etc. Therefore it is imperative that any oral electrolyte solution used to resuscitate calves contain an alkalinizing agent.
Acetate, propionate, and bicarbonate are all considered alkalinizing agents. Bicarbonate is commonly available in oral electrolyte solutions. Recent research has shown that acetate and propionate containing oral electrolyte solutions are preferred over bicarbonate for several reasons:
Acetate and propionate produce energy when metabolized, whereas bicarbonate does not.
Acetate and propionate do not increase abomasa pH whereas bicarbonate does.
Acetate and propionate are volatile fatty acids and can facilitate sodium and water absorption in the calf small intestine whereas bicarbonate does not.
Acetate and propionate inhibit the growth of Salmonella species.
Acetate and propionate don’t alkalinize the abomasum or
interfere with milk clotting in the abomasum, whereas bicarbonate does.
Several pathogenic bacteria are killed at a low pH, for example both E. coli and Salmonella are killed at a pH around 3.0 and begin to multiply at a pH above 5.5. Normally the stomach (abomasum) maintains a very low (acidic) pH which is critical for decreasing the number of pathogenic bacteria reaching the small intestine and increasing the resistance to intestinal colonization by bacteria. More simply stated, the calf needs to maintain a low abomasa pH to decrease the incidence of infection and clinical disease.
Recent research has shown oral electrolyte solutions containing bicarbonate induce a significant increase in abomasa pH for a prolonged period of time which may increase the number of bacteria that are able to colonize the small intestine. This effect is not observed when using acetate based oral electrolyte solutions. Therefore abomasa and small intestinal alkalinisation due to bicarbonate-containing oral electrolytes may promote bacterial growth, and actually prolong or worsen the diarrhoea in calves.
Even with the possible drawbacks associated with using bicarbonate, it is still critical that your oral electrolyte solution contain an alkalinizing agent. While they may not be ideal, products containing only bicarbonate have been used effectively for years to resuscitate calves and will likely be used for years to come. However, there are several products on the market that do not contain any of the three alkalinizing agents listed above and should not be used in calves. These products may correct dehydration and electrolyte abnormalities, however they will not have any ability to increase blood pH (correct the acidosis) which is one of our primary therapeutic goals.
A calf that has normal electrolyte levels (i.e. sodium, calcium, potassium) may still very likely die of acidosis if this is not addressed. Therefore always make sure either bicarbonate, acetate, or propionate are listed on the ingredients list of the oral electrolyte product you are using, and if an analysis is present, the minimum recommended concentration for an alkalinizing agent would be 50 to 60 mM/L (lower concentrations are likely to have a very weak alkalinizing ability).
The ratio between sodium and chloride is important in improving a calf’s acid-base status. The normal ratio of sodium to chloride in blood plasma is about 4:3 (140 mmol/L sodium:103 mmol/L chloride). Using sodium chloride as our only source of these two ions provides one chloride ion for every sodium, slightly overrepresenting chloride in terms of normal plasma concentrations.
To achieve an appropriate ratio of sodium to chloride, especially if we want to have a chance at correcting acidosis, ingredients other than sodium chloride e.g. sodium bicarbonate, sodium acetate and sodium lactate (“alkalinizing” or “buffering” agents) must also be used in the formula.
When selecting an electrolyte, look for alkalinizing agents which include a combination of bicarbonate, propionate and acetate.
Glucose/dextrose:
How much glucose/dextrose should be in an electrolyte? When a calf is sick, it will naturally pull any energy available to help combat what’s making it ill, putting the calf at risk for lower average daily weight gain. When a calf is suffering from dehydration, it’s in need of an energy source to correct hypoglycaemia and negative energy balance.
Glucose in an electrolyte provides a minor energy source for the calf. An electrolyte solution should not be looked at as a replacement for energy provided by milk or milk replacer which is why it is recommended to keep a calf on a milk ration during times of illness.
High glucose electrolyte solutions are sometimes presented and used as a replacement for milk or milk replacer during diarrhoea. Five – six litres of such a solution provides about 75% of the daily energy needed by a baby calf for maintenance, while providing none of the protein required by the calf. However electrolytes with very high levels of glucose/dextrose can bring harm to calves by slowing down gut movement, which could cause bloat. If a calf already has scours, it can worsen their symptoms.
Glucose, which is absorbed more quickly than lactose (milk sugar), causes a rapid increase in plasma glucose. Insulin is released into the calf’s bloodstream to lower the elevated plasma glucose level. This insulin response is excessive in young calves. Within three hours after administration of the high glucose electrolyte solution, plasma glucose is lower than the pre-treatment level.
Lactose is the principal carbohydrate found in milk. It is a disaccharide containing one molecule each of monosaccharides (simple sugars) glucose and galactose and is the primary carbohydrate source for neonatal mammals. During the digestive process lactose is broken down into its component glucose and galactose sub-units by the enzyme lactase and is then absorbed from the digestive tract for use by the body. As the process of absorption occurs over a longer time period the rapid increase in plasma glucose associated with glucose is reduced. The presence of lactose in the gut also increases the population of Lactobacillus sp. and Bifidobacterium sp in the intestines. …these are favourable bacteria populations.
All good electrolytes contain a mix of glucose for rapid energy uptake and lactose for sustained energy uptake.
Gelling agents:
The rationale for including gelling agents in an electrolyte is that they may:
increase the viscosity of the solution, resulting in a decreased rate of stomach emptying,
slow the passage and distribution of the solution to the small intestine, thus increasing the potential for absorption of nutrients, and
provide a "coating" effect on inflamed intestinal mucosa.
Trials conducted in the US using one such product (Advance Arrest) which utilizes Guar gum as a gelling agent concluded that the rate of passage through the digestive tract was slower for the electrolyte solution containing guar gum, and that absorption of sugars from the intestine also occurred more slowly. Decreasing the rates of passage and absorption could be beneficial for calves with diarrhoea, by increasing the time that nutrients contact the absorptive surface of the intestine and by providing a more constant influx of nutrients into the body.
The “must haves” of
an ideal electrolyte:
Geof Smith, DVM, MS, PhD, North Carolina State University, suggests the following “must haves” for the ideal oral electrolyte.
Must have enough sodium (90 – 130mmo/L) to help correct extracellular fluid deficits
Must have glycine (amino acid) or acetate, to help facilitate intestinal sodium absorption
Must have an alkanising agent (acetate, propionate, bicarbonate) to help correct metabolic acidosis
Must have sufficient energy (glucose) especially if calves will be off milk for a day or two
Must have components that will not increase the chance of bacterial infection and support a healthy gastrointestinal environment
Must have an osmolality <650 mOsm/L (ideally between 400 – 600 mOsm/L) to ensure timely emptying of the abomason/stomach to avoid abomasal bloat due to fermentation of produst slow in passing due to high osmolality
Must have a strong ion difference of at least 60 (cations relative to anions)
In addition to his list of “must haves” he also suggests five goals for treating calf scours:
Rehydration
Correction of acidosis
Correction of electrolyte abnormalities (Na, K, Cl)
Reversal of negative energy balance
Inhibit the growth of pathogenic bacteria.
Summary:
There are tremendous variations in the formulations of oral electrolyte solutions currently available in the New Zealand. Table 4 shows an analysis of ten popular products and gives some comments on their suitability for use in calves. Not all of these products would be ideal and in fact a few would not be recommended at all. It is important to work closely with your veterinarian to select the product that is most appropriate for your herd to optimize your calf diarrhoea treatment protocols.
BIOCALF Plus Provides energy, nutrients, minerals, probiotics, prebiotic yeast, enzymes and gelling agent Guar gum.
BIOCALF Restore. Provides energy, nutrients, and minerals, at a low cost
SITES OF INTEREST
Antahi Innovations Ltd Antahi Innovations Ltd are a company that specialises in innovative calf rearing solutions
Calf Notes Read the latest international research and information on calf rearing?
Calf Sessions A resource for calf health, nutrition, physiology and management
NZAGBIZ From colostrum management to health and biosecurity, experts from AgResearch and Fonterra discuss all things calf rearing.
Electrolyte comparison table:
Click on the link below to download Table 4
Download the Electrolyte product comparison table
References: Penstate University College of Agricultural Science https://extension.psu.edu Geof Smith, DVM, MS, PhD, North Carolina State University. https://www.researchgate.net Rob Costello, Technical Specialist, Calf Sessions, https://calfsessions.com Use of Gelling Agents in Electrolyte Solutions for Calves. http://livestocktrail.illinois.edu
Download the Understanding Electrolyte Product Comparisons. PDF